Dive into the fascinating world of lithium battery electrolytes with our comprehensive guide! Uncover the types of electrolytes used, their crucial role in battery performance, and factors influencing conductivity. Discover maintenance tips for extending electrolyte lifespan and glimpse into future developments in lithium battery electrolyte technology. Whether you’re a battery enthusiast or seeking essential knowledge, buckle up for an illuminating journey into the heart of lithium battery technology!
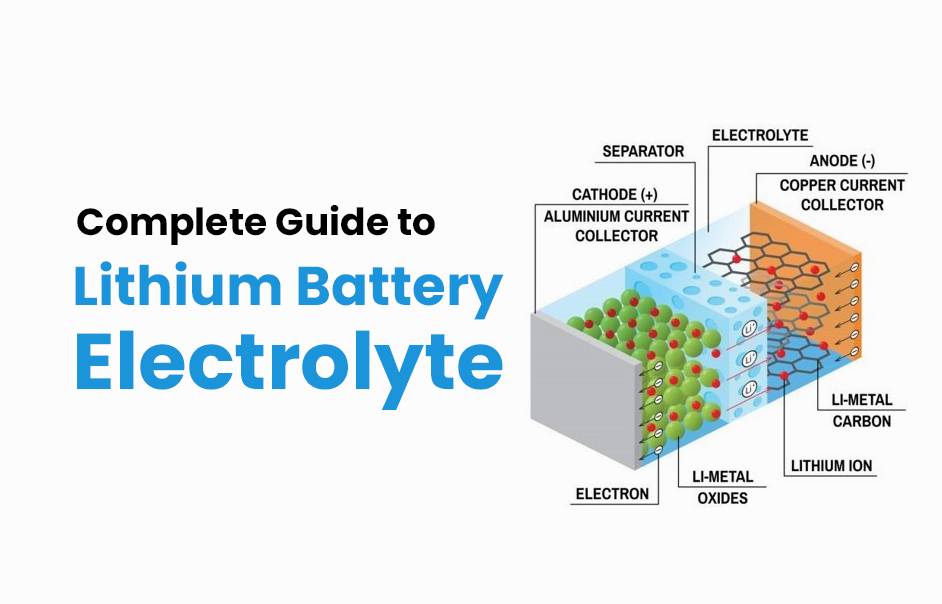
Types of Electrolytes used in Lithium Batteries

Lithium batteries have become a popular choice for various applications due to their high energy density and long lifespan. One crucial component of these batteries is the electrolyte, which plays a vital role in their performance.
There are several types of electrolytes used in lithium batteries, each with its own unique characteristics. The most common type is liquid electrolyte, which consists of a lithium salt dissolved in an organic solvent. This type offers good conductivity and low cost but can be prone to leakage and safety issues.
Solid-state electrolytes are another option that has gained attention in recent years. These electrolytes are made up of solid materials that conduct ions, eliminating the need for a liquid medium. Solid-state electrolytes offer improved safety and stability compared to liquid ones.
Polymer-based gel or gel polymer electrolytes provide a compromise between liquid and solid-state options. They combine the advantages of both by offering higher conductivity than solid-state alternatives while still providing improved safety compared to traditional liquid electrolytes.
Other types include ceramic-based and glass-based electrolytes, which offer excellent stability at high temperatures but may have lower conductivity.
There is no one-size-fits-all solution when it comes to choosing an electrolyte for lithium batteries. Each type has its own strengths and weaknesses, depending on the specific requirements of the application at hand. Ongoing research aims to develop new materials that can further enhance battery performance while prioritizing safety and sustainability considerations
Importance of Electrolyte in Battery Performance
The electrolyte is a crucial component in the performance of lithium batteries. It plays a vital role in facilitating the movement of ions between the battery’s electrodes, enabling the flow of electricity. Without an effective electrolyte, the battery’s overall performance would be severely compromised.
One important aspect to consider is the conductivity of the electrolyte. A higher conductivity allows for more efficient ion movement and thus improves battery performance. This is why researchers are constantly striving to develop electrolytes with enhanced conductivity.
Another key factor is stability. The electrolyte must be chemically stable to ensure long-term reliability and prevent degradation over time. Unstable electrolytes can lead to reduced capacity, decreased efficiency, or even safety issues within lithium batteries.
Moreover, temperature sensitivity should not be overlooked when considering the importance of electrolytes in battery performance. Some types of electrolytes may experience significant changes in their properties at extreme temperatures, affecting overall battery functionality.
Additionally, compatibility between the electrode materials and the chosen electrolyte is critical for optimal performance. Mismatched components can lead to undesired reactions that decrease energy output or shorten battery lifespan.
In conclusion (as requested), it becomes clear that selecting an appropriate and high-quality electrolyte significantly impacts lithium battery performance by influencing factors such as conductivity, stability, temperature sensitivity, and compatibility with other materials used in batteries’ construction
Factors Affecting the Conductivity of Lithium Battery Electrolyte
The conductivity of the electrolyte plays a crucial role in determining the performance and efficiency of lithium batteries. Several factors can impact the conductivity, ultimately affecting the overall battery performance.
One key factor is temperature. The conductance of electrolytes is highly dependent on temperature, with higher temperatures generally resulting in better conductivity. However, extreme temperatures can also have adverse effects on battery life and safety.
Another important factor is the concentration of salts in the electrolyte solution. Higher salt concentrations typically lead to increased ionic conductivity due to more available charge carriers. However, excessively high concentrations can result in reduced stability and potential issues with electrode reactions.
The choice of solvents used in the electrolyte formulation is yet another influential factor. Different solvents have varying dielectric constants and viscosities, which directly affect ion mobility within the system. Choosing appropriate solvents that balance these properties is essential for optimal conductivity.
Electrolyte additives are also worth considering as they can significantly impact conductivity. Additives like conductive polymers or nanoparticles can enhance ion transport and increase overall ionic conduction through various mechanisms.
Additionally, contamination or impurities present in either the electrodes or electrolyte itself can detrimentally affect conductivity by interfering with ion movement or causing side reactions.
Understanding these factors that influence electrolyte conductivity allows researchers to develop new materials and designs for improved lithium battery performance. By optimizing these parameters, we can maximize energy storage capabilities while ensuring long-term durability and safety.
Maintenance and Care for Lithium Battery Electrolyte

Proper maintenance and care of the electrolyte in lithium batteries is crucial to ensure optimal performance and longevity. Here are some important tips to keep in mind:
1. Temperature control: It is essential to store and operate lithium batteries within a suitable temperature range. Extreme heat or cold can affect the electrolyte’s conductivity, leading to reduced battery efficiency.
2. Avoid overcharging or deep discharging: Overcharging or deeply discharging a lithium battery can cause irreversible damage to both the active materials and the electrolyte. This can result in decreased capacity and potential safety hazards.
3. Regular inspections: Periodic visual inspections of your lithium battery can help identify any signs of leakage, corrosion, or other issues with the electrolyte. If any problems are detected, it is advisable to seek professional assistance promptly.
4. Proper handling: When handling lithium batteries, always follow manufacturer guidelines regarding storage, transportation, and installation procedures. Mishandling can lead to damage that affects not only the electrolyte but also overall battery performance.
5. Cleanliness matters: Keep your lithium battery clean by wiping off any dirt or contaminants from its surface regularly. Contaminants may interfere with proper functioning of the electrolyte and compromise its conductivity.
By following these maintenance practices, you can maximize the lifespan and efficiency of your lithium battery system while ensuring safe operation.
Future Developments in Lithium Battery Electrolyte Technology
As the demand for more efficient and sustainable energy storage solutions continues to grow, researchers and scientists are constantly exploring innovative developments in lithium battery electrolyte technology. These advancements aim to enhance the performance, safety, and longevity of lithium batteries.
One area of focus is the development of solid-state electrolytes. Traditional liquid electrolytes can be volatile and flammable, posing safety risks. Solid-state electrolytes offer a safer alternative by eliminating the need for a liquid component. They also have potential benefits such as increased energy density and improved stability at higher temperatures.
Another promising direction in electrolyte technology is the incorporation of additives that improve battery performance. Additives such as conductive polymers or redox mediators can enhance ion conductivity, reduce internal resistance, and increase overall efficiency. By fine-tuning these additives, researchers hope to optimize battery performance while maintaining cost-effectiveness.
Furthermore, efforts are being made to develop highly stable electrolytes that minimize degradation over time. This would result in longer-lasting batteries with reduced capacity loss during cycling. Additionally, improving the thermal stability of electrolytes could help prevent overheating issues often associated with high-performance lithium batteries.
Moreover, research is ongoing into new materials for lithium battery electrodes that can interact efficiently with the chosen type of electrolyte. The compatibility between electrode materials and an electrolytic solution plays a crucial role in determining overall battery performance.
In conclusion (not included), future developments in lithium battery electrolyte technology hold great promise for revolutionizing energy storage systems across various industries – from electric vehicles to renewable energy grids – by offering greater efficiency, enhanced safety features,and extended lifespan capabilities.
What are the components of lithium batteries?
How do lithium batteries work?
Conclusion
Lithium battery electrolyte plays a crucial role in the performance and longevity of lithium batteries. With its ability to conduct ions between the cathode and anode, the electrolyte is responsible for enabling the flow of electricity and maintaining stable operation.
In this complete guide to lithium battery electrolyte, we’ve explored different types of electrolytes used in lithium batteries, including liquid and solid-state options. We’ve also discussed the importance of electrolyte in battery performance, highlighting its impact on capacity, efficiency, and safety.
FAQs
How Does Electrolyte Modification Prevent Overcharge?
Why Lithium-Ion Batteries Prone to Overcharge Issues?
Due to their specific chemistry and design, lithium-ion batteries have a higher energy density and are more sensitive to overcharging compared to other battery types. Overcharging can lead to excess energy release, causing the battery to overheat, degrade, and become unstable. The internal structure of lithium-ion batteries, including the delicate electrolyte, makes them vulnerable to overcharge-related problems. Understanding these factors helps in implementing proper charging practices for lithium-ion batteries.