The battery’s evolution, from Alessandro Volta’s voltaic pile to the modern lithium-ion battery, has revolutionized technology and daily life. Volta’s invention in 1800 laid the foundation, while the 20th-century development of lithium-ion batteries by Goodenough, Whittingham, and Yoshino transformed consumer electronics, transportation, and renewable energy storage. Batteries power everything from smartphones to electric vehicles, shaping our modern world.
When and Who Invented the Battery?
The journey of the battery began centuries ago. Alessandro Volta, an Italian physicist, is credited with inventing the first true battery in 1800. His invention, known as the voltaic pile, consisted of alternating layers of zinc and copper discs separated by cardboard soaked in saltwater. This device produced a steady flow of electric current and laid the foundation for modern battery technology.
The Birthplace of the Battery
Alessandro Volta, Volta’s groundbreaking invention was born in the town of Como, located in present-day Italy. It was here that Volta conducted his experiments and unveiled his revolutionary voltaic pile to the world.

Exploring the Purpose Behind Battery Invention
The invention of batteries was driven by a desire to harness and store electrical energy for practical applications. Volta and other early scientists sought to understand electricity and its potential uses, leading to the development of batteries as a reliable source of power for various devices and systems.
The Origin of Battery Naming
The term “battery” originated from the military, where multiple artillery pieces were grouped together to create a powerful force. Similarly, early batteries consisted of multiple cells connected in series to increase voltage and power output, resembling a military battery in structure and function.
Understanding the Lithium-Ion Battery
Fast forward to the late 20th century, and the lithium-ion battery emerged as a game-changer in energy storage technology. Unlike traditional batteries, which rely on chemical reactions between metals and electrolytes, lithium-ion batteries use lithium ions to store and release energy, offering higher energy density and longer lifespan.
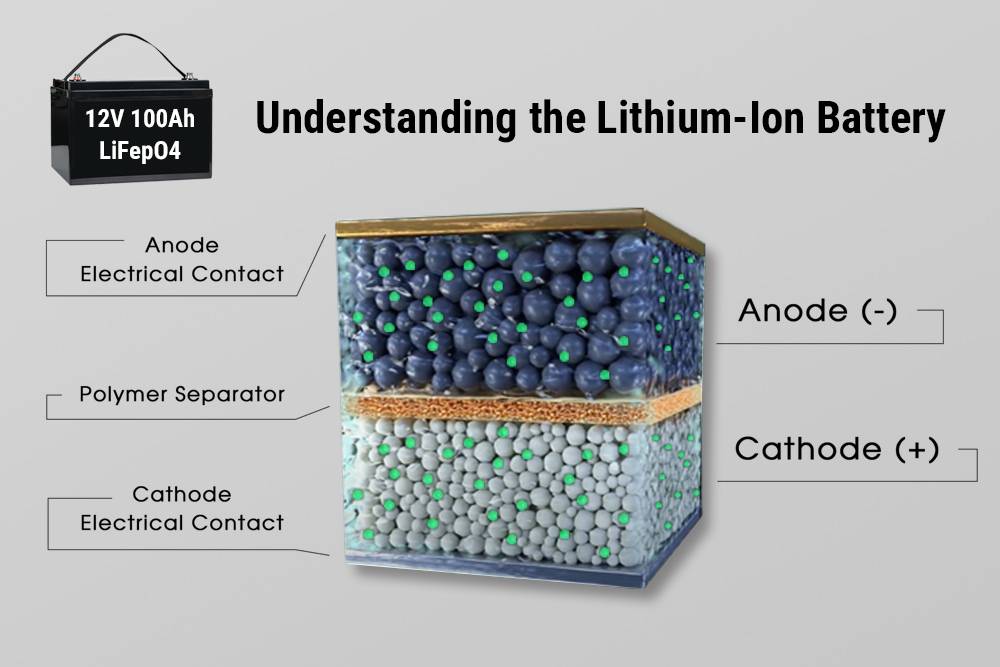
The Origins of the Lithium-Ion Battery
The development of the lithium-ion battery can be traced back to the 1970s when researchers John Goodenough, Stanley Whittingham, and Akira Yoshino made significant breakthroughs in lithium battery technology. Their work led to the commercialization of lithium-ion batteries in the 1990s, revolutionizing consumer electronics, transportation, and renewable energy storage.
Unraveling the Motivation Behind Lithium Battery Invention
The motivation behind inventing the lithium-ion battery stemmed from the need for lightweight, high-energy-density power sources for portable electronic devices. Traditional batteries, such as nickel-cadmium and nickel-metal hydride, were heavy and had limited capacity, making them unsuitable for emerging technologies like smartphones and laptops.
Considering the Consequences of a World Without Batteries
Imagine a world without batteries. Our modern way of life would be vastly different. We rely on batteries to power everything from smartphones and laptops to electric vehicles and renewable energy systems. Without batteries, we would lose the convenience and mobility that these devices provide, and our ability to harness clean, renewable energy sources would be severely limited.

In conclusion, the invention and evolution of batteries have transformed the way we live, work, and interact with the world around us. From Alessandro Volta’s voltaic pile to the revolutionary lithium-ion battery, each milestone in battery technology has brought us closer to a more sustainable and connected future.