LTO (Lithium Titanate) batteries find applications in electric vehicles, renewable energy storage systems, grid energy storage, and industrial applications requiring high power and fast charging capabilities. Their robust performance, long cycle life, and ability to operate in extreme temperatures make them suitable for demanding applications.
Advantages of LTO Batteries
LTO (Lithium Titanate) batteries offer several advantages, including high power density, long cycle life, fast charging capability, wide temperature range operation, and enhanced safety features. These advantages make LTO batteries a preferred choice for various applications.

Disadvantages of LTO Batteries
LTO (Lithium Titanate) batteries have certain disadvantages, including lower energy density, higher cost, and a narrower range of available sizes and capacities. However, these drawbacks are outweighed by the battery’s advantages in terms of high power density, long cycle life, fast charging capability, and enhanced safety features.
Applications and Uses of LTO Batteries
LTO (Lithium Titanate) batteries find applications in electric vehicles, renewable energy storage systems, grid energy storage, and industrial applications requiring high power and fast charging capabilities. Their robust performance, long cycle life, and ability to operate in extreme temperatures make them suitable for demanding applications.

Maintenance and Care for LTO Batteries
Comparison with Other Types of Batteries
Lithium titanate batteries (LTO) have become a focal point in recent years due to their exceptional features. Notably, their extended cycle life, rapid charging, and safety advantages set them apart in various applications. Let’s explore these key aspects.

- Extended Cycle Life: LTO batteries surpass traditional lithium-ion batteries with an impressive cycle life, exceeding 10,000 cycles. This longevity makes them perfect for applications requiring frequent charging, ensuring lasting reliability.
- Fast Charging Capability: Unlike batteries with lengthy charging times, LTO batteries can reach 80% capacity in minutes. This rapid charging is especially beneficial for electric vehicles and energy storage systems, minimizing downtime.
- Excellent Safety Profile: LTO batteries, in contrast to other lithium-ion types, boast a stellar safety record. Their minimal risk of thermal runaway or combustion enhances their dependability across various applications.
- Wide Operating Temperature Range: LTO batteries stand out due to their efficiency in extreme temperatures (-50°C to +70°C), making them suitable for diverse weather conditions and ensuring reliable performance.
- Low Self-Discharge Rate: With a low self-discharge rate, LTO batteries retain charge over extended periods of inactivity. This makes them ideal for backup power systems or devices with intermittent usage patterns.
Limitations: While LTO batteries offer numerous advantages, it’s essential to consider their limitations. One notable drawback is their lower energy density compared to some lithium-ion cells, impacting overall energy storage capacity. Additionally, the higher cost of LTO batteries should be taken into account when evaluating their suitability for specific applications.
In conclusion, LTO batteries present a compelling choice for various applications, excelling in longevity, charging speed, safety, temperature resilience, and low self-discharge. However, understanding their limitations, such as energy density and cost, is crucial for informed decision-making in specific use cases.
Future Developments and Innovations in LTO Battery Technology
As technology advances, lithium titanate (LTO) batteries are undergoing continuous improvements to cater to diverse industry needs. Key areas of focus include enhancing energy density, reducing charging time, extending cycle life, ensuring safety, and exploring sustainable manufacturing processes.
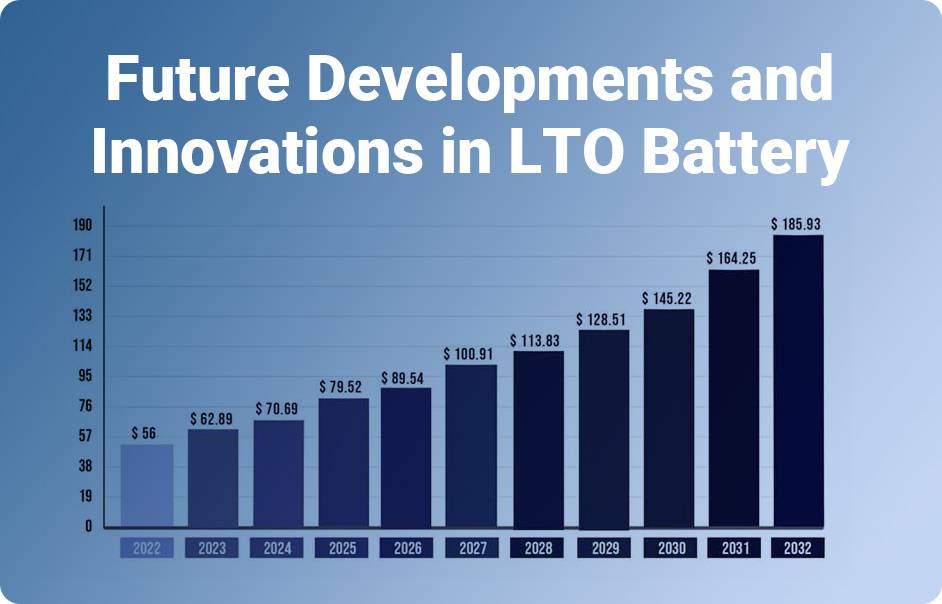
- Increased Energy Density: Future developments target boosting the energy density of LTO batteries. This improvement aims to store more energy efficiently within a smaller space, enhancing their appeal for applications requiring prolonged power.
- Reduced Charging Time: Researchers are working on further minimizing the charging time of LTO batteries. Optimizing battery chemistry and implementing advanced charging algorithms seek to make these batteries even more valuable in industries where swift rechargeability is essential.
- Improved Cycle Life: Efforts are directed towards enhancing the cycle life of LTO batteries. Extending the number of charge-discharge cycles will contribute to their longevity, making them more cost-effective over time compared to some other lithium-ion technologies.
- Enhanced Safety Features: Ongoing research prioritizes the safety of LTO batteries. Scientists are exploring ways to improve thermal stability and prevent issues like overheating or thermal runaway, ensuring the secure and reliable operation of these batteries.
- Sustainable Manufacturing: Researchers are exploring eco-friendly alternatives for manufacturing LTO batteries. The focus is on developing sustainable processes to minimize the environmental impact associated with battery production and reduce waste generation.
In conclusion, ongoing research holds the promise of expanding the capabilities of Lithium Titanate Batteries (LTO). The future envisions increased energy density, reduced charging times, improved cycle life, enhanced safety features, and sustainable manufacturing practices, ensuring a more efficient and environmentally friendly battery technology.
Conclusion
Explore the realm of Lithium Titanate Batteries (LTO) with this guide, unveiling their safety, fast charging, and applications like electric vehicles. Despite limitations such as lower energy density and higher costs, LTO batteries excel in reliability. Ongoing research promises enhanced performance, making LTO a compelling choice for longevity-focused applications. Whether opting for LTO or alternatives, grasp the advantages, drawbacks, and maintenance essentials to make informed decisions in the dynamic landscape of advanced energy storage.
FAQs
How can you care for and manage starter batteries?
Taking care of starter batteries is crucial for maintaining their performance and longevity. It is important to regularly check the battery’s health, ensure proper charging, and prevent sulfation. Giving batteries a second life through recycling is also an eco-friendly approach. Additionally, understanding impedance spectroscopy and monitoring the battery’s capacity can help in effective management. Techniques such as examining loading characteristics and improving the battery fuel gauge can further enhance the overall care and management of starter batteries.

What happened to the old comments from the previous website, and how can users access them?
The old comments from the previous website are not compatible with the new commenting system. However, they have been preserved for users’ reference and use. Users can access the preserved old comments by selecting the option to “Show Old Comments” within the new commenting system.
Can I cite the article in my thesis or academic work, and if so, what reference information is available?
Both Erhan and Engineerhan asked if they could cite the article in their theses and requested more specific reference information. They expressed appreciation for the content and indicated a desire to properly reference it in their academic works.
Is there a comprehensive summary of different battery technologies in the article?
The article provides a thorough overview of various battery technologies, acknowledging the fast-paced nature of the industry with ongoing research and development efforts by numerous scientists in both academic and corporate settings. The article also suggests the need for updates due to the dynamic nature of the field, indicating that the last update was conducted three years prior.
What are the details provided in the article regarding lithium cell technologies and chemistry?
The article discusses various battery technologies and chemistries, focusing on lithium cell technologies in particular. It highlights the fast-evolving nature of this market, with research scientists from universities and corporations actively developing new variations of these chemistries. The author suggests updating the article to reflect these ongoing advancements, indicating that the last update was made three years ago.
How can I refer to the article in my own work or publication?
To refer to the article in your own work or publication, you can include relevant information such as the date of the comment, the name of the commenter (Ashrith Domun), as well as the specific topic being discussed regarding NTO (Lithium-titanium Niobium) and its comparison to other materials like LTO and LFP in terms of specific energy and costs. This reference helps provide context for readers and supports your discussion of the topic.
What are the advantages and disadvantages of future battery technologies like solid-state Li-ion, lithium-sulfur, and lithium-air batteries?
Future battery technologies such as solid-state Li-ion, lithium-sulfur, and lithium-air batteries offer unique advantages and disadvantages in terms of energy storage capabilities and performance.
Solid-state Li-ion batteries provide high specific energy levels, which is beneficial for maximizing energy storage capacity. However, they face challenges in terms of loading efficiency and safety concerns.
Lithium-sulfur batteries also offer high specific energy, allowing for increased energy density. However, they suffer from issues related to cycle life longevity and loading capabilities.
Lithium-air batteries boast high specific energy levels as well but face challenges in terms of loading efficiency and require clean air for optimal operation. Additionally, they have a shorter lifespan compared to other battery technologies.
In conclusion, each of these future battery technologies offers the advantage of high specific energy but comes with its own set of disadvantages such as poor loading efficiency, safety concerns, short lifespan, and cycle life limitations. Understanding these trade-offs is crucial in determining the most suitable battery technology for specific applications.
What are the differences in specific energy, power, and thermal stability among different lithium-based battery systems?

The comparison of different lithium-based battery systems reveals unique characteristics in specific energy, power, and thermal stability. Li-aluminum (NCA) stands out for its high specific energy capacity, while Li-manganese (LMO) and Li-phosphate (LFP) excel in specific power and thermal stability. On the other hand, Li-titanate (LTO) may have lower capacity but surpasses other systems in terms of lifespan and cold temperature performance. As the focus shifts to electric powertrains, safety and cycle life become more critical factors than capacity alone.
What are the key characteristics of Lithium Nickel Cobalt Aluminum Oxide (NCA) batteries?
Lithium Nickel Cobalt Aluminum Oxide (NCA) batteries possess key characteristics that define their performance and usage. These batteries feature a LiNiCoAlO2 cathode with around 9% cobalt content and a graphite anode. This particular battery technology, abbreviated as NCA or Li-aluminum, has been in existence since 1999.
In terms of voltages, NCA batteries have a nominal voltage of 3.60V and typically operate within a range of 3.0V to 4.2V per cell. The specific energy or capacity of these batteries falls between 200-260Wh/kg, with an expected capacity of up to 300Wh/kg. When it comes to charging, NCA batteries have a charge rate of 0.7C, reaching a full charge at 4.20V (in most cells) with a standard charge time of around 3 hours; however, fast charging is achievable with certain cell variations. It is important to cease charging when the current saturates at 0.05C to prevent any issues.
During discharge, NCA batteries typically operate at 1C, with a cut-off at 3.00V. High discharge rates can impact the battery’s lifespan, shortening it significantly. These batteries offer a cycle life of around 500 cycles, a metric that is influenced by factors such as depth of discharge and operating temperature. Thermal runaway, a critical concern in battery safety, is typically triggered at around 150°C (302°F), and high charging rates can exacerbate this issue.
In terms of cost, NCA batteries are priced at approximately $350 per kilowatt-hour. These batteries find applications in various industries such as medical devices, industrial settings, and electric powertrains (notably used by Tesla). They exhibit similarities to Li-cobalt batteries, are classified as Energy Cells, and are primarily produced by Panasonic and utilized by companies like Tesla, signifying significant growth potential in the battery market. This detailed insight into the characteristics of Lithium Nickel Cobalt Aluminum Oxide batteries sheds light on their capabilities, limitations, and applications in various fields.
What are the key characteristics of Lithium Iron Phosphate (LFP) batteries?
Lithium Iron Phosphate (LFP) batteries are known for their stable performance and safety features. These batteries have a nominal voltage range of 3.20 to 3.30V, with an operating range of 2.5 to 3.65V per cell. They offer a specific energy capacity of 90 to 120Wh/kg and are typically charged at a 1C rate. The charging process should be stopped when the current saturates at 0.05C to prevent any damage.
During discharge, LFP batteries can handle high load currents, with some cells capable of up to 25C discharge rate and a 40A pulse for 2 seconds. These batteries have a cycle life of 2000 cycles or more, depending on factors such as depth of discharge and temperature. Additionally, LFP batteries are considered very safe even when fully charged, with a thermal runaway temperature of 270°C.
In terms of cost, LFP batteries are relatively affordable at around $580 per kilowatt-hour. They are commonly used in portable and stationary applications that require high load currents and endurance. The batteries exhibit a very flat voltage discharge curve and are widely regarded as one of the safest options among lithium-ion batteries.
Overall, LFP batteries are suitable for applications in special markets, especially those focused on energy storage due to their moderate growth and elevated self-discharge characteristics.
How has the performance of these battery technologies evolved over time, as indicated by the updates provided in the passage?
Over time, the performance of the mentioned battery technologies has seen significant changes. They have transitioned to emphasizing high power delivery while having reduced capacity compared to earlier versions. A key point is the enhanced safety profile relative to lithium-cobalt batteries. Additionally, these batteries are often combined with NMC to boost their overall performance. The passage suggests that, given their characteristics and the current market landscape, these technologies may not have the same growth potential as in the past.
What are the advantages and disadvantages of using Lithium Iron Phosphate (LiFePO4) batteries?
Lithium Iron Phosphate (LiFePO4) batteries offer several advantages, including excellent electrochemical performance with low resistance, high current rating, and long cycle life. They also provide good thermal stability, enhanced safety, and tolerance to abuse. LiFePO4 batteries are more tolerant to full charge conditions and prolonged high voltage exposure compared to other lithium-ion systems. However, a trade-off for these benefits is their lower nominal voltage of 3.2V/cell, which decreases their specific energy below cobalt-blended lithium-ion batteries. LiFePO4 batteries have a higher self-discharge rate than other Li-ion batteries, which can lead to balancing issues over time. Despite this, LiFePO4 batteries are commonly used to replace lead acid starter batteries due to their excellent safety features and long lifespan, even though they have moderate specific energy and elevated self-discharge rates.
How does the combination of nickel, manganese, and cobalt in Lithium Nickel Manganese Cobalt Oxide (NMC) contribute to its performance?
The combination of nickel, manganese, and cobalt in Lithium Nickel Manganese Cobalt Oxide (NMC) enhances the performance of the compound through a synergistic effect. Nickel boasts high specific energy but lacks stability on its own, while manganese is distinguished for its ability to form a spinel structure, which results in low internal resistance but with a trade-off of lower specific energy. By combining these different metals, the resulting NMC compound leverages the strengths of each element to compensate for the weaknesses of the others. The presence of cobalt also plays a crucial role in enhancing the overall performance of NMC. This combination creates a compound that offers a balanced mix of specific energy, stability, and internal resistance, making it an excellent choice for various energy storage applications.
What are the characteristics of Lithium Manganese Oxide (LMO) and its applications?
Lithium Manganese Oxide (LMO), also known as LiMn2O4, is a cathode material with a spinel structure that has been utilized in various applications since 1996. This material operates within a voltage range of 3.0 to 4.2V per cell, with a nominal voltage of 3.70V (3.80V). LMO offers a specific energy capacity of 100-150Wh/kg and can be charged at rates ranging from 0.7 to 1C, with a maximum charging rate of 3C. Charging is typically stopped when the current saturates at 0.05C. In terms of discharge, LMO can operate at 1C, with certain cells supporting rates of up to 10C and pulse rates of 30C for short durations. The cutoff voltage for discharge typically stands at 2.50V.
The cycle life of LMO batteries ranges from 300 to 700 cycles and is influenced by factors such as depth of discharge and temperature. Thermal runaway, a concern with high-energy batteries, is observed at around 250°C and is often linked to aggressive charging strategies. Despite its high power output and safety advantages over lithium-cobalt batteries, LMO is characterized by lower overall capacity. To enhance performance, LMO is frequently combined with Nickel Manganese Cobalt Oxide (NMC).
In terms of applications, Lithium Manganese Oxide finds utility in power tools, medical devices, and electric powertrains. While LMO’s power capabilities are valued, its growth potential is somewhat restricted, and newer technologies are emerging that may offer improved characteristics.
How do Li-manganese batteries improve specific energy and life span when blended with lithium nickel manganese cobalt oxide (NMC)?
Li-manganese batteries enhance their specific energy and extend their lifespan when combined with lithium nickel manganese cobalt oxide (NMC) by effectively blending the benefits of each system. This combination optimizes the performance of the battery, particularly in electric vehicles like the Nissan Leaf, Chevy Volt, and BMW i3. The inclusion of LMO (NMC) in the battery composition, typically making up around 30% of the overall structure, contributes a significant high current boost during acceleration due to its inherent characteristics. Meanwhile, the NMC component of the blend enhances the battery’s longevity and provides a longer driving range. Research in the field of Li-ion batteries often focuses on integrating Li-manganese with elements such as cobalt, nickel, manganese, and aluminum to serve as active cathode materials, ultimately contributing to improved specific energy and overall lifespan of the batteries.
What are the applications and advantages of Li-manganese batteries in comparison to Li-cobalt?
Li-manganese batteries find applications in various industries including power tools, medical instruments, hybrid and electric vehicles due to their unique characteristics. When compared to Li-cobalt batteries, Li-manganese batteries offer a capacity that is slightly lower but provide greater flexibility in design. Engineers can tailor the battery to maximize either its longevity, load current, or capacity, depending on the specific application requirements. Additionally, Li-manganese batteries often combine with lithium nickel manganese cobalt oxide (NMC) to enhance specific energy and extend the battery’s life span. In terms of advantages over Li-cobalt batteries, Li-manganese batteries deliver improvements in specific power, safety features, and overall life span.
How does Lithium Manganese Oxide (LiMn2O4) differ from Lithium Cobalt Oxide in terms of structure and performance?
Lithium Manganese Oxide (LiMn2O4) differs from Lithium Cobalt Oxide in terms of both structure and performance. Structurally, LiMn2O4 has a capacity that is approximately one-third lower than Li-cobalt. However, the design flexibility of LiMn2O4 allows engineers to optimize the battery for either optimal longevity, maximum load current, or high capacity. In terms of performance, Li-manganese offers improvements in specific power, safety, and lifespan when compared to Li-cobalt. Additionally, Li-manganese batteries can be blended with lithium nickel manganese cobalt oxide (NMC) to enhance specific energy and extend lifespan effectively. The combination of LiMn2O4 and NMC brings out the best in each system, making it an ideal choice for electric vehicles such as the Nissan Leaf, Chevy Volt, and BMW i3.
What are the specific energy, specific power, safety, life span, and cost factors associated with Lithium Cobalt Oxide batteries?
Lithium Cobalt Oxide (LCO), also known as LiCoO2, is a type of battery chemistry commonly used in devices like mobile phones, tablets, laptops, and cameras. LCO batteries offer a specific energy capacity of 150-200Wh/kg, with some specialty cells providing up to 240Wh/kg. This high specific energy makes LCO batteries desirable for applications where energy density is crucial.
In terms of specific power, LCO batteries have limited capabilities compared to other types of batteries. They are typically charged at a rate of 0.7-1C, reaching a voltage of 4.20V. It is important to note that charging LCO batteries above 1C can shorten the battery’s lifespan. Additionally, it is recommended to turn off the charging process when the current saturates at 0.05C to avoid any potential safety issues.
When it comes to the lifespan of LCO batteries, they are expected to have a cycle life of around 500-1000 cycles. The actual cycle life can be influenced by factors such as the depth of discharge, the applied load, and the operating temperature. Proper usage and maintenance can help maximize the lifespan of LCO batteries.
In terms of safety, LCO batteries are known to have a thermal runaway temperature of around 150°C (302°F). It is important to note that allowing the battery to reach full charge can promote thermal runaway, which is a critical safety concern. Monitoring the battery’s temperature during charging and discharging processes is crucial to ensure safe operation.
Lastly, the cost factor associated with LCO batteries is influenced by the presence of cobalt in the cathode material. Cobalt is relatively expensive, which can impact the overall cost of LCO batteries compared to other types of chemistries. As of an update in 2019, the market share of LCO batteries has stabilized, indicating a consistent demand despite the cost factor associated with cobalt.
How does Li-cobalt compare to other Lithium-ion chemistries like Li-manganese, NMC, and NCA?
Li-cobalt batteries are becoming less popular when compared to other Lithium-ion chemistries such as Li-manganese, NMC, and NCA. This shift is primarily driven by the high cost of cobalt and advancements in performance achievable through blending with other active cathode materials. In the case of Li-manganese batteries, they often incorporate lithium nickel manganese cobalt oxide (NMC) to enhance specific energy levels and prolong the overall lifespan of the battery. This blending approach is favored because it allows each material to complement the strengths of the other, resulting in improved battery efficiency and longevity. Notably, lithium nickel manganese oxide (NMC) is frequently chosen for electric vehicles like the Nissan Leaf, Chevy Volt, and BMW i3 due to its optimal performance characteristics. Ongoing research in the field of Li-ion batteries is focused on combining Li-manganese with cobalt, nickel, manganese, and/or aluminum to further enhance the capabilities of these batteries, highlighting the continuous evolution and innovation in battery technology.
What are the limitations and considerations for charging and discharging Li-cobalt batteries?
When it comes to charging and discharging Li-cobalt batteries, it is important to adhere to specific limitations and considerations to ensure their longevity and safe operation. These batteries should not be charged or discharged at rates higher than their C-rating, as doing so can lead to overheating and unnecessary strain on the battery. For instance, a Li-cobalt battery with a capacity of 2,400mAh should ideally only be charged or discharged at a rate not exceeding 2,400mA.
To achieve optimal fast-charging results while maintaining the battery’s health, it is advised to adhere to the manufacturer’s recommendation of a C-rate of 0.8C, which translates to around 2,000mA. This controlled charging rate helps prevent excessive heat buildup and extends the overall lifespan of the Li-cobalt battery. By being mindful of these limitations and considerations during charging and discharging processes, users can effectively preserve the performance and safety of their Li-cobalt batteries.
How does the structure of Li-cobalt batteries work during discharge and charge?
In Li-cobalt batteries, the structure plays a critical role in the discharge and charge processes. During discharge, lithium ions travel from the anode to the cathode, while during charging, this flow is reversed from the cathode to the anode. The cathode of these batteries typically features a layered structure that facilitates the movement of lithium ions back and forth during these processes. This specific design allows for efficient energy transfer and storage within the battery, enabling it to function effectively during discharge and charge cycles.
What are the characteristics, advantages, and drawbacks of Lithium Cobalt Oxide (LiCoO2)?
Lithium Cobalt Oxide (LiCoO2), commonly known as Li-cobalt, is known for its high specific energy, making it a popular choice for devices such as mobile phones, laptops, and digital cameras. While it excels in providing high specific energy, it offers only moderate performance in terms of specific power, safety, and life span. The Li-cobalt battery is composed of a cobalt oxide cathode and a graphite carbon anode, featuring a layered structure in the cathode.
However, Li-cobalt does have its drawbacks. One significant limitation is its relatively short life span, along with low thermal stability and limited load capabilities. It is crucial to note that Li-cobalt batteries should not be charged or discharged at a current higher than its designated C-rating to prevent overheating and undue stress. Furthermore, Li-cobalt is gradually losing popularity to alternatives such as Li-manganese, NMC, and NCA due to the high cost of cobalt and the improved performance achieved by blending Li-cobalt with other active cathode materials.
What are the chemical symbols and abbreviations for different Lithium-ion chemistries?
Lithium-ion chemistries use chemical symbols and abbreviations to represent their active materials. For instance, lithium cobalt oxide is commonly known as LiCoO2 with the abbreviation LCO. This chemistry can also be simplified as Li-cobalt due to the primary active material being cobalt. Similarly, other lithium-ion chemistries are also designated with short-form names based on their chemical symbols and abbreviations.
What are the types of Lithium-ion batteries commonly used?
The types of Lithium-ion batteries commonly used include Lithium Cobalt Oxide (LiCoO2) which is also known as LCO, and Lithium Manganese Oxide (LiMn2O4) which is referred to as LMO.
Which type of lithium cell technology is considered the safest
Redway ongoing research prioritizes the safety of LTO batteries, with scientists exploring methods to enhance thermal stability and prevent issues like overheating or thermal runaway, thus ensuring secure and reliable battery operation. Additionally, Lithium Iron Polymer (LFP) has been identified as the safest type of lithium cell technology. Redway engineers remarked, “Looks like Lithium Iron Polymer LFP is the safest of them all,” emphasizing the significance of safety advancements in lithium cell technologies for reliable battery operation.
What is the self-discharge rate of the LTO battery stored at 20°C for 90 days?
How many cycles can the LTO battery last for and what are its charging and discharging characteristics?
Why is Lithium Titanate technology considered the future of today?
What safety tests has the LTO battery passed?







